Archimica at a Glance - Strengths
We provide quality products and services, consistently, reliably responsibly and continuously applying
some of the world’s most difficult to handle chemical technologies.
Discover our last Company Profile.
Download here
Experience with the Most Challenging Reagents, Reactions and Substance Classes Known.

Made in
Europe
Agile Capacity
Availability

Premium Client
Attention
Highly Flexible Manufacturing
and Development
History Lodi Site
Archimica’s Evolution – 77 Years of Continuity of Supply into the World´s Pharmaceutical Market.
1947
Foundation of Istituto Chemioterapico Italiano (I.C.I.)
1984
Foundation of Prime European Therapeuticals S.p.A (Euticals)
2008
Mandarin Capital Partners becomes majority Euticals shareholder
2012
Clessidra, an Italian Financial Fund becomes majority Euticals shareholder
2016
AMRI acquired Euticals Group
2019
Effective August 1, Livia acquires Lodi site, and brands it Archimica S.p.A.
2023
PI Health Sciences acquires Archimica S.p.A.

Products
APIs/GMP Building Block – Process Development and Manufacturing.
From Clinical to Large Commercial Scale for the Innovative and Generic Industry.
APIs, GMP Building blocks, Reagents
Customers are relying on Archimica’s Products and Services for Quality Medicine Worldwide.
All Therapeutic Classes. All Substance Classes (including Anticancer APIs)
7
Active Products
24
Active Drug Master Files in 39 Countries
200
Active Customers
50
Supplied Countries Worldwide
Generic API Portfolio - Active Pharmaceutical Ingredient
- Cytarabine (nucleoside analogue) – CEP
- Hydroxyurea (modified urea) – CEP
- Mianserin (tetracyclic) – CEP
- Sucralfate (saccharose sulfate)
- Sulfathiazole (sulfonamide)
- Sucrose octasulfate potassium salt (KSOS) -non-API grade
- Glycerophosphoryl-Choline (GPC) – technical grade
- Glycerophosphorylethanolamine (GPE) – technical grade
- Bromfenac – EUDMF/USDMF
- Capecitabine – EUDMF/USDMF
- Citicoline – EUDMF
- Dihydralazinesulfate – EUDMF
- Disopyramide – EUDMF
- Hydralazine hydrochloride – EUDMF/USDMF
- Lithium Carbonate – EUDMF/USDMF
- Pirenzepine Dihydrochloride – EUDMF/USDMF
- Sulfapyridine – EUDMF/USDMF
- Thenfadil – EUDMF
- Tolmetin – EUDMF
- Oxcarbazepine (dibenzazepine) – EUDMF/USDMF
Technologies & Plant
Staff
58%
PRODUCTION
22%
QUALITY
10%
MAINTENANCE
10%
SALES & ADMINISTRATION
Plant
Manufacturing Overview
Reactor Capacity Supported by a Vast Range of Sophisticated Ancillary Technology:
Reactor Volume: About 250 m3 (stainless steel and glass line).
Temperature Range: -20 to 160°.
Pressure Range: 10 mbar to 6 bar.
Reactor Volume Range: 50 to 10.000 liters.
Dryers: Fluid bed dryer, agitated vacuum pan dryer, vacuum horizontal agitated dryer, vacuum double cone dryer, Planex® dryer, tray dryer.
Centrifuges/Filters
Filters: Filter Presses – Filter Dryers – Pressure Filters
Centrifuges: Vertical Axis top discharge, Vertical Axis Bottom Discharge, Horizontal Axis Peeler.
Ancillary Equipment: Sieves – Ultra Filters, Ion Exchange Chromatography Columns.
Kilo Lab – 500 L – Scale up/filing products
Products plant list
F1 – QC Laboratory
F2A – Multi-Purpose Plant
F3A – Raw Materials, Intermediates and APIs warehouse
F3B – Finishing & Drying Plant (ISO 8, controlled environment)
F5A – Hydroxyurea Plant
F5B – Multi-Purpose Plant
F5C – Cytarabine Plant
F6A – Sucralfate Plant
F6B – Sulfamidics Plant
F7 – Multi-Purpose Plant
F8 – Utilities
Tech
Main differentiators
Highly flexible and broad permit for all technologies, reagents and solvents.
Broad range of extraordinary hazardous chemistry and chemicals processable in large scale:
solvents, including chlorinated and toxic solvents;
Strong acids including phosphorous oxychloride, chlorosulfonicacid, hydrofluoric acid;
Highly flammable, highly corrosive, explosive chemicals.
Residual thermal oxidizer allowing trace gas contamination removal, all waste gas streams.
Seveso guideline compliant.
Own on-site waste water treatment, backed by external partners.
Solvent recovery and tank farms.
Differentiators - Technology specialities
Large Scale Deacylated Phospholipids production (GPC, GPE)
Large Scale Use of Pyridine as reagent or solvent, as well as pyridine-SO3 complex handling.
Cytostatics manufacturing/drying: Nucleosides.
Heterocyclic chemistry: Pyridines, Piperazines, Quinoxalines, Quinolones, Quinazolinones, Benzofuranes.
Chromatographic purification with ionic resins.
Micronization (third party partners).
Up to OEB4 Potency Level.
Differentiators - Reagents & Solvents
Chlorosulfonic acid
Sulfur trioxide
Pyridine
Ethylene oxide
Triphosgene
Hydrazine, Azides, Diphenyl-phosphorylazide, Acetyl Nitrate
Halogen chemistry:
Fluorine, chlorine, bromine, iodine
Hydrofluoric acid
Chlorine, chlorinating agents
Bromine, brominating agents (NBS, dibromohydantoin, phosphorous bromides)
Methylene diiodide, methyliodide, iodoform, TMSOZ
Kilo Lab
Capable of handling: 1 to 10kg batch size
Three stages of manufacturing –
Glass Radley reactors ; SS reactors –
Filtration & drying –
Powder processing
Safety
Safety
IPPC-based Authorization.
Integrated Pollution Prevention and Control.
Highly flexible and broad permit for all technologies, reagents and solvents.
Compliance with IED Directive 2010/75/UE.
Best Available Technology.
Compliance with Directive 105/15 2012/18/EU.
Seveso Directive.
Prevention and Control of On-Shore Major Accident Hazards involving Dangerous Substances.
Quality System, Inspections, Certificates
Quality System Based on EU GMP part II, ICH Q7.
Quality Processes Handled via TrackwiseTM.Deviations, Out-of-Specifications (OOS), CAPA, Change Control.
US/EU Drug Master Files. 24 Active Drug Master Files in 39 Countries and 4 active CEP.
API Registration Form: AMA -50/2020
GMP Certificate (Human): IT- API/1H/2025
GMP Certificate (Veterinary): NBF/3/2025/V
FEI Number 3002874947
DUNS Number 429351620

US-FDA
Last inspection April 2017

AIFA
Last Inspection October 2024

KFDA (Korea)
Last inspection October 2011

Veterinary Ministry of Health
Last inspection November 2024
Procurement
Archimica is continuously strengthening its Suppliers base.
Please contact our Procurement Department using our contact form below if you want to be part of our Archimica journey.
News
Discover our latest news and articles about
products, research and services.
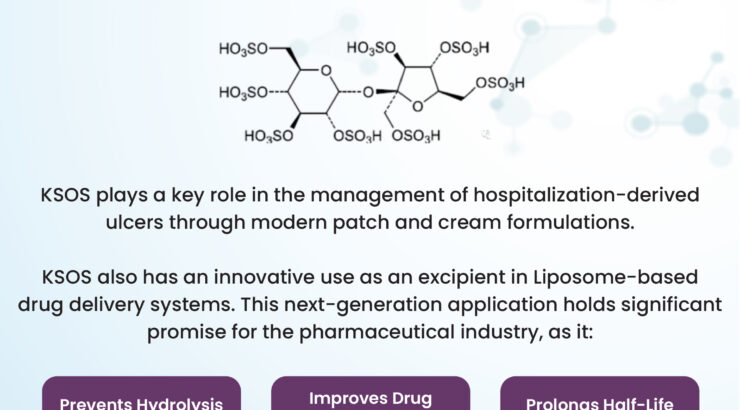
Sucrose Octasulfate Potassium Salt (KSOS)
NewsAt Archimica, we're thrilled to explore the transformative potential of Sucrose Octasulfate Potassium Salt (KSOS) in revolutionizing pharmaceutical applications. From enhancing the stability of high-value treatments to improving liposome-based drug delivery systems,Read moreDCAT Week 2025
Events NewsArchimica is excited to be a part of DCAT Week 2025, where we'll be showcasing our latest breakthroughs and innovations in pharmaceutical manufacturing. Let's connect and discuss how we canRead moreMianserin is now CEP Certified
Events NewsWe are happy to announce that Archimica is now CEP (Certification of Suitability) certified by the EDQM (European Directorate for the Quality of Medicines & HealthCare) for our API product,Read more
Contacts
GPS coordinates
Latitude: 45 °19’ 25,3“ N
Longitude: 9°27’ 57,7’’ E
The site is located in Northern Italy in Lombardia about 30 km South of Milano and Linate airport. Access to the facility is the motorway A1 Milano-Bologna and the route SS9 Via Emilia.
Site address
Viale Milano 86,
26900 Lodi (LO) Italy
Phone: +39 0371 49021
Fax: +39 0371 610019
Email: contact@archimica.com
We appreciate your interest in Archimica.
For any inquiries please contact us